Scuba diving, a thrilling and awe-inspiring activity, allows individuals to explore the mesmerizing world beneath the ocean's surface. However, this adventure wouldn't be possible without a fundamental understanding of the physical laws governing the underwater environment. One such law, Boyle's Law, plays a pivotal role in the world of scuba diving, influencing pressure underwater and shaping the safety and mechanics of the sport.
Boyle's Law Unveiled:
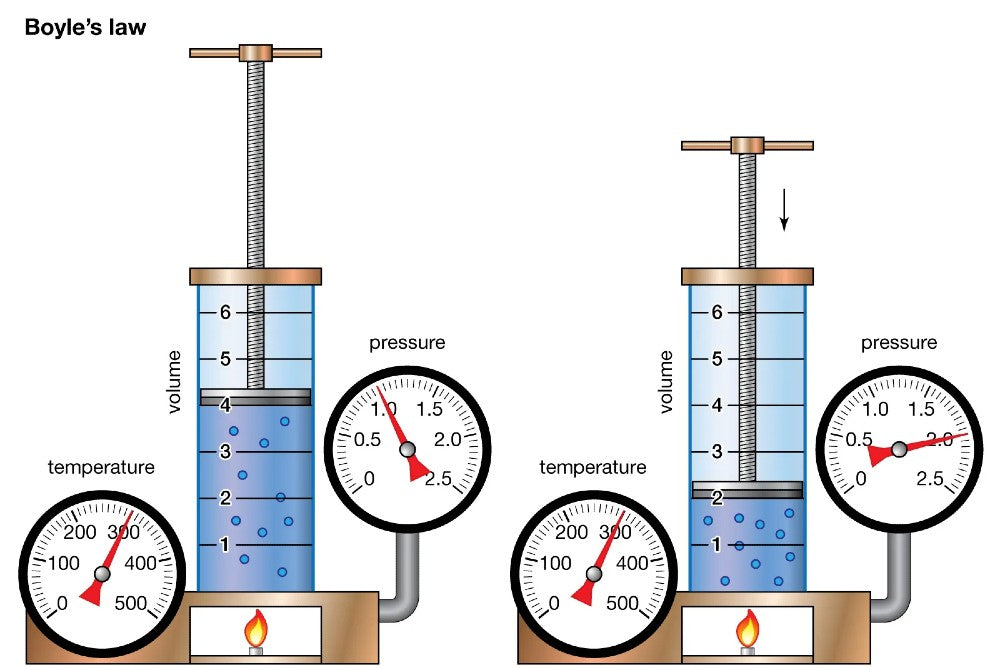
Named after physicist Robert Boyle, Boyle's Law describes the relationship between the pressure and volume of a gas at a constant temperature. The law is expressed mathematically as P1V1 = P2V2, where P represents pressure and V stands for volume. In simpler terms, when the volume of a gas decreases, the pressure it exerts increases proportionally, and vice versa, provided the temperature remains constant.
Applying Boyle's Law to Scuba Diving:
Scuba diving involves breathing air supplied by a tank carried on the diver's back. As a diver descends underwater, Boyle's Law becomes a crucial factor in understanding the changes in pressure and volume of the breathing gas inside the scuba tank.
-
Descending and Increasing Pressure: As a diver descends into the depths of the ocean, the ambient pressure surrounding them rises significantly. Boyle's Law explains that as the pressure increases, the volume of the gas within the scuba tank decreases. This means that the air a diver breathes at the surface occupies more space in the tank than the same amount of air at greater depths.
-
Equalizing Pressure: Equalizing pressure is vital to prevent barotrauma, a condition caused by pressure changes. Boyle's Law comes into play when equalizing the pressure in various air-containing spaces in the body, such as the ears and sinuses. Divers adjust the volume of these air spaces by inhaling or exhaling to maintain equilibrium with the increasing ambient pressure as they descend.
-
Air Consumption and Tank Management: Understanding Boyle's Law is crucial for managing air consumption and dive time. The reduction in gas volume due to increased pressure means that divers consume air more rapidly at depth. Divers must carefully monitor their air supply and plan their ascent to avoid running out of breathing gas.
The Impact of Boyle's Law on Scuba Equipment:
Boyle's Law not only affects the gas within the diver's body but also plays a crucial role in the design and functionality of scuba equipment.
-
Regulators and Pressure: Scuba regulators are designed to deliver air to the diver at ambient pressure, regardless of the depth. Boyle's Law is integral to the regulator's function, as it ensures that the air supplied to the diver is at the appropriate pressure for the given depth.
-
Buoyancy Control Devices (BCDs): Boyle's Law is a key factor in the design of BCDs, which help divers control their buoyancy. As a diver ascends, the decreasing pressure causes the gas in the BCD to expand. This expansion results in increased buoyancy, and divers must release air from the BCD to maintain a controlled ascent.
-
Dive Tables and Dive Computers: Dive tables and dive computers take Boyle's Law into account when calculating dive profiles and decompression limits. These tools help divers plan their dives by considering the impact of pressure changes on the absorption and elimination of inert gases in the body.
Safety Measures and Boyle's Law:
Understanding Boyle's Law is not only essential for optimizing dive experiences but also for ensuring the safety of divers. Ignoring the principles of Boyle's Law can lead to various risks, including barotrauma, decompression sickness, and other pressure-related injuries.
-
Ascent Rate and Safety Stops: Boyle's Law emphasizes the importance of a controlled ascent to allow the body to off-gas safely. Divers follow recommended ascent rates and include safety stops during their ascent to minimize the risk of decompression sickness.
-
Pressure-Related Equalization Techniques: Divers employ equalization techniques, such as the Valsalva maneuver, to equalize pressure in the ears and sinuses. Ignoring these techniques could lead to ear injuries and discomfort.
-
Gas Narcosis Awareness: As depth increases, the pressure affects the partial pressure of gases in the breathing mixture. Nitrogen, a component of air, can cause narcosis at greater depths. Divers must be aware of the potential for nitrogen narcosis and take appropriate precautions.
Conclusion:
Boyle's Law is a fundamental concept in physics that finds real-world application in the realm of scuba diving. Its influence on pressure, volume, and gas behavior underwater shapes the safety protocols, equipment design, and overall experience of divers. By delving into the intricacies of Boyle's Law, divers can deepen their understanding of the underwater environment, ensuring that each descent into the ocean's depths is both exhilarating and safe.